Description
Test de antígeno GENEDIA W COVID-19 Ag Nasopharyngeal and sputum – Pack 20
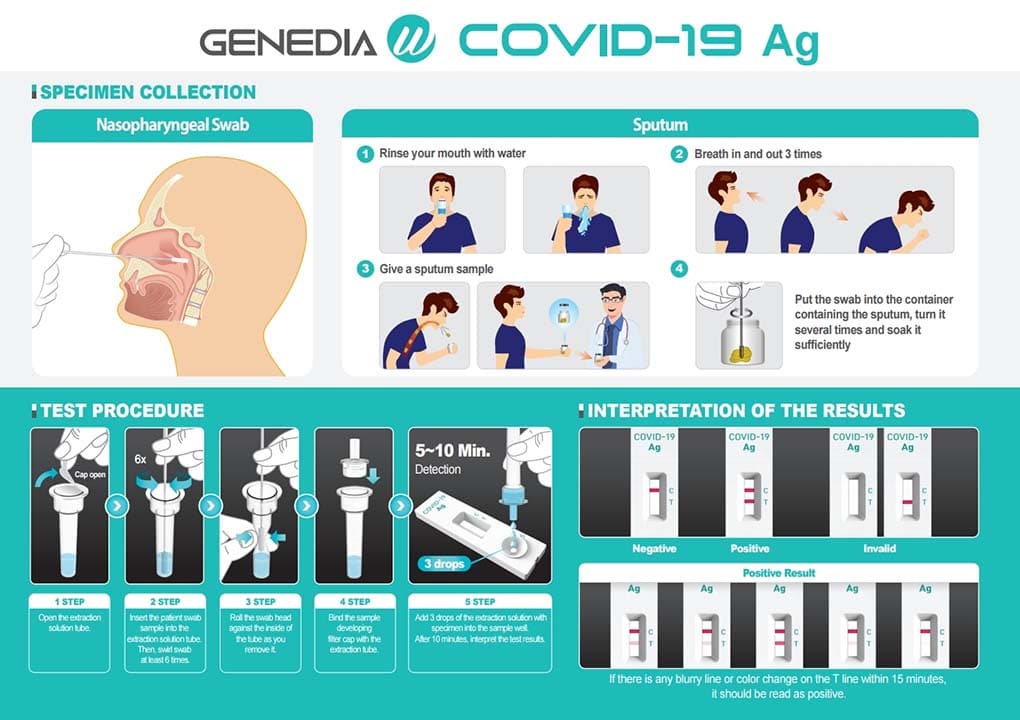
The SarS-CoV-2 GENEDIA W COVID-19 Ag is a colorimetric lateral flow immunochromatographic assay. The test contains antibodies specific to the SARS-CoV-2 antigen on the test line (T). If the SARS-CoV-2 antigen is present in the biological sample, a visible black band appears on the test line (T) as a result of the formation of the antigen-antibody complex. The control line (C) is used for procedure control and should always appear if the test is correctly performed.
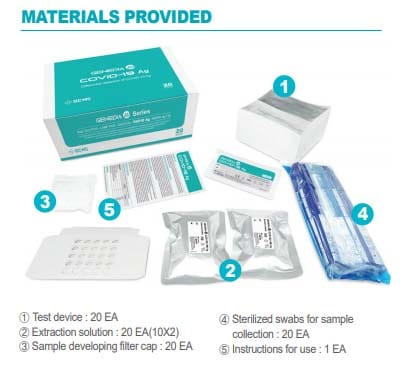
Its use is intended for the qualitative detection of the SARS-CoV-2 antigen from a sample, taken with a swab of nasopharyngeal exudate or sputum / saliva with rapid results in 10 minutes and with high levels of sensitivity and specificity.
The state-of-the-art tests from the prestigious Korean manufacturer Genedia W COVID-19 Ag allow the collection of the sample by both methods while maintaining high levels of sensitivity in both cases. This makes the task easier especially when it is executed in pediatric, geriatric, psychiatric or other similar centers that require a less invasive application.
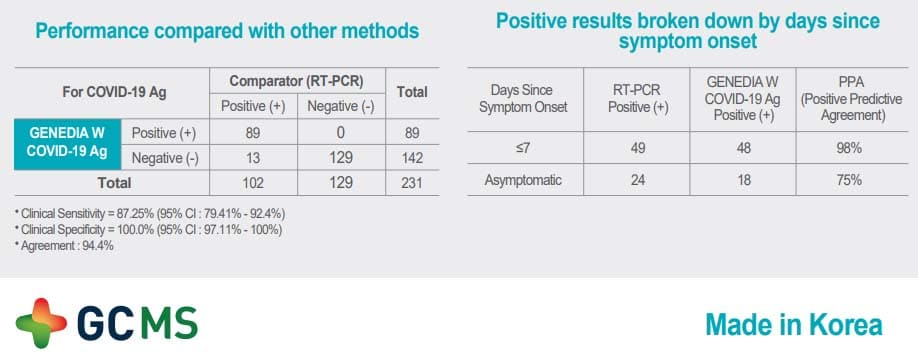
This new GENEDIA W COVID-19 Ag test is one of the most advanced in the market. It offers the highest sensitivity levels and at the moment is one of the few approved for dual testing methods – both nasopharyngeal and saliva.
La prueba rápida de antígeno GENEDIA W COVID-19 Ag ofrece una solución rápida y fiable para la detección del de antígeno de SARS-CoV-2 en aplicaciones remotas y sin necesidad de instrumental de laboratorio a un coste muy eficiente.